Abstract
Introduction: Vasoactive intestinal peptide (VIP) is a 28-amino acid neuropeptide with immunosuppressive effects on T cells. Inhibition of VIP receptor (VIP-R) signaling by VIPhyb, a first-generation VIP-R antagonist, not only enhances T-cell activation and proliferation in vitro but also improves T cell dependent anti-tumor response in mouse models of acute myeloid leukemia (AML) and T lymphoblastic leukemia (Li et al. 2016; Petersen, Li, and Waller 2017). The goal of the project is to develop more potent VIP-R antagonists that generate a significantly more robust anti-tumor response in mouse models of AML, when compared to VIPhyb and validate a screening method to test the efficacy of novel peptides in activating human T cells in vitro. In this study, we report, for the first time, the activity of novel VIP-R antagonists on the activation profile of human T cells.
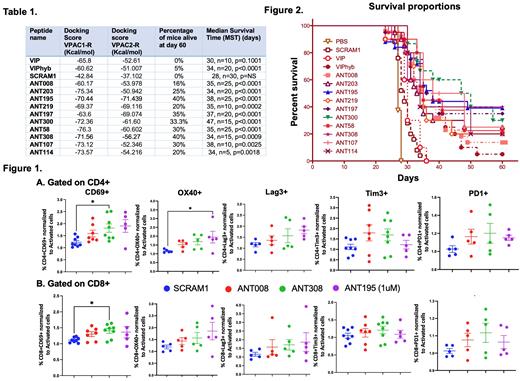
Methods: We utilized in-silico-based modeling to identify 10 novel VIP-R antagonists from a library of 300 peptide sequences predicted to have increased binding affinity to VIP receptors VPAC1 and VPAC2 when compared to VIP or VIPhyb (Table 1). The library was generated from peptide sequences that contain the six charged N-terminal residues of the neurotensin present in VIPhyb with two or more amino acid substitutions within the C-terminal amino acid sequence of VIP. The ability of these peptides was tested in vitro using T cells from multiple healthy human donors activated using anti-CD3 monoclonal antibody coated plates. Activation status was assessed by flow cytometry of CD69, OX40, PD1, Tim3 and Lag3 expression relative to control cultures without added peptides. Potency of the novel antagonists in vivo was tested in a mouse AML model, by treating C1498- bearing mice with subcutaneous administration of VIP, VIPhyb, scrambled peptide (SCRAM1) or the second-generation VIP-R antagonists (labeled as 'ANT') from day 6-12 after tumor implantation.
Results: Inhibiting VIP-R signaling in human T cells using second-generation VIP-R antagonists ANT008, ANT308 and ANT195 showed approximately 1.5-to-2-fold increase in CD69, OX40, Tim3, Lag3 and OX40 expression in CD4+ T cells following 24-hour of drug exposure compared to control cultures (Figure 1A). A smaller effect of VIP-R antagonists on activation of CD8+ subsets was observed (Figure 1B). Among the peptides, ANT195 was superior to ANT008 and ANT308 which shows potency even at 1μM compared to 3μM for ANT008 and ANT308. However, significant increase in CD69 expression was observed in both CD4+ and CD8+ T cells in cultures treated with ANT308 (Figure 1 A&B, *p<0.05). Viability of the T cells was not affected by incubation with the queried peptides (Data not shown). These data corresponded to in vivo activity of the novel VIP-R antagonists such as ANT308 and ANT195 which rendered 40% of mice leukemia-free at day 60 compared to only 5% long-term survival with VIPhyb (Figure 2). Another candidate, ANT300, increased median survival time (MST) by up to 47 days compared to MST of 34 days with VIPhyb (Figure 2).
Conclusions: Here, we report a simple and robust in vitro method to screen for immune activity potential of novel second-generation VIP-R antagonists using human T cells. Preliminary screen shows VIP-R antagonists augment activation of both CD4+ and CD8+ T cells. Our results indicate that ANT308 and ANT195 are more potent VIP-R antagonists with enhanced activity in vitro (human) and in vivo (mouse) than VIPhyb and ANT008, which demonstrate lower predicted binding affinities to VPAC1 and VPAC2. Our study supports the hypothesis that higher predicted binding affinity to VPAC1 and/or VPAC2 is associated with enhanced activity in stimulating human T cells and promoting anti-leukemia activity in mice. Further mechanistic studies on how inhibition of VIP-R signaling augments T cell activation and function are underway. These novel antagonists can lead to peptide-based immunotherapy for the treatment of various liquid cancers. Clinical development of this novel concept will require appropriate pre-clinical pharmacokinetic and toxicology studies.
Waller: Cambium Oncology: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Verastem Oncology: Consultancy, Research Funding.